12th Annual Risk
Management and Pharmacovigilance
Summit
6 – 7 May, 2026
Berlin

Speakers
Summit will host speakers from the world’s leading companies

Yuri van Geertruij
Executive Director, Sub-Regional Lead for Mid-Sized Markets


Friederike Hoffgen
Area Safety Head Germany & CE


Alex Bica
Global Head Early Development and Translational Safety


Anna Kończak
EU QPPV


Ghazi El Masri
Quality Auditor, Senior Manager


Ahmed Nasr
Operations Director Egypt and Director Milk Africa & Turkey


Khaled Elsharkawy
Integrated Supply Chain Senior Director


Mueen Uddin Siddique
Senior Director – Access Network Procurement


Chamath Nawaratne
VP & CPO Middle East &Africa


Shannon Hore
Senior Vice President Procurement and Logistics


Sandeep Sharma
Director Group Procurement & International Markets Supply Chain

Hottest topics
More details about what will be discussed this annual
New Lessons Learned
New Opportunities Unlocked
New Lessons Learned
New Opportunities Unlocked


Look at the Main Topics
Of Our Conference
- 01Inspection trends in 2026: What regulators now focus on first
- 02From document to living framework: Operationalising RMPs
- 03New expectations for signal detection timelines and documentation
- 04Metrics that matter: Beyond ICSR submission timelines
- 05Data integrity findings in PV: Lessons learned the hard way
- 06Cross-functional ownership: Who controls RWE in PV?
- 07Transparency in safety decision-making
- 08From operational execution to strategic safety leadership
Early bird tickets available
Month of early booking discounts
Discount
10 %
Discount
20 %
Discount
0 %
Testimonials
What our users say about our conferences
Our Clients
"We partner with top professionals to provide creative ideas, inspiration and guidance"






























Sponsors
Our trusted sponsors


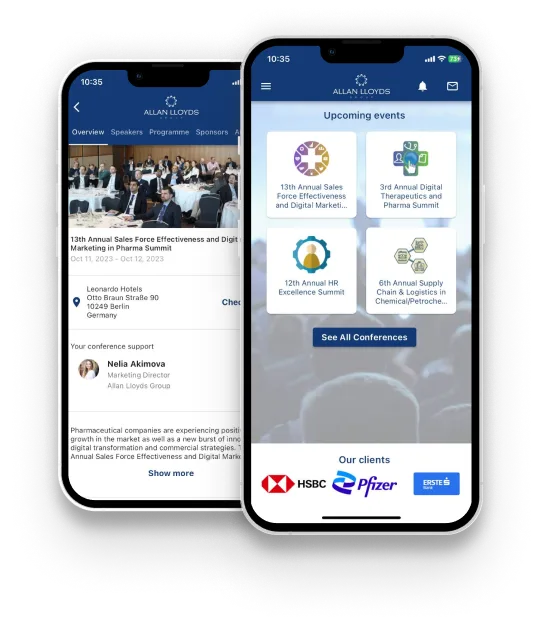
Download our App to
Enhance Your Event Experience
This innovative app is aimed at enhancing your networking experience through a seamless blend of interaction and connectivity. We have carefully crafted this application with new features that empower you to make the most out of your engagements, ensuring personalised experiences and seamless networking opportunities.